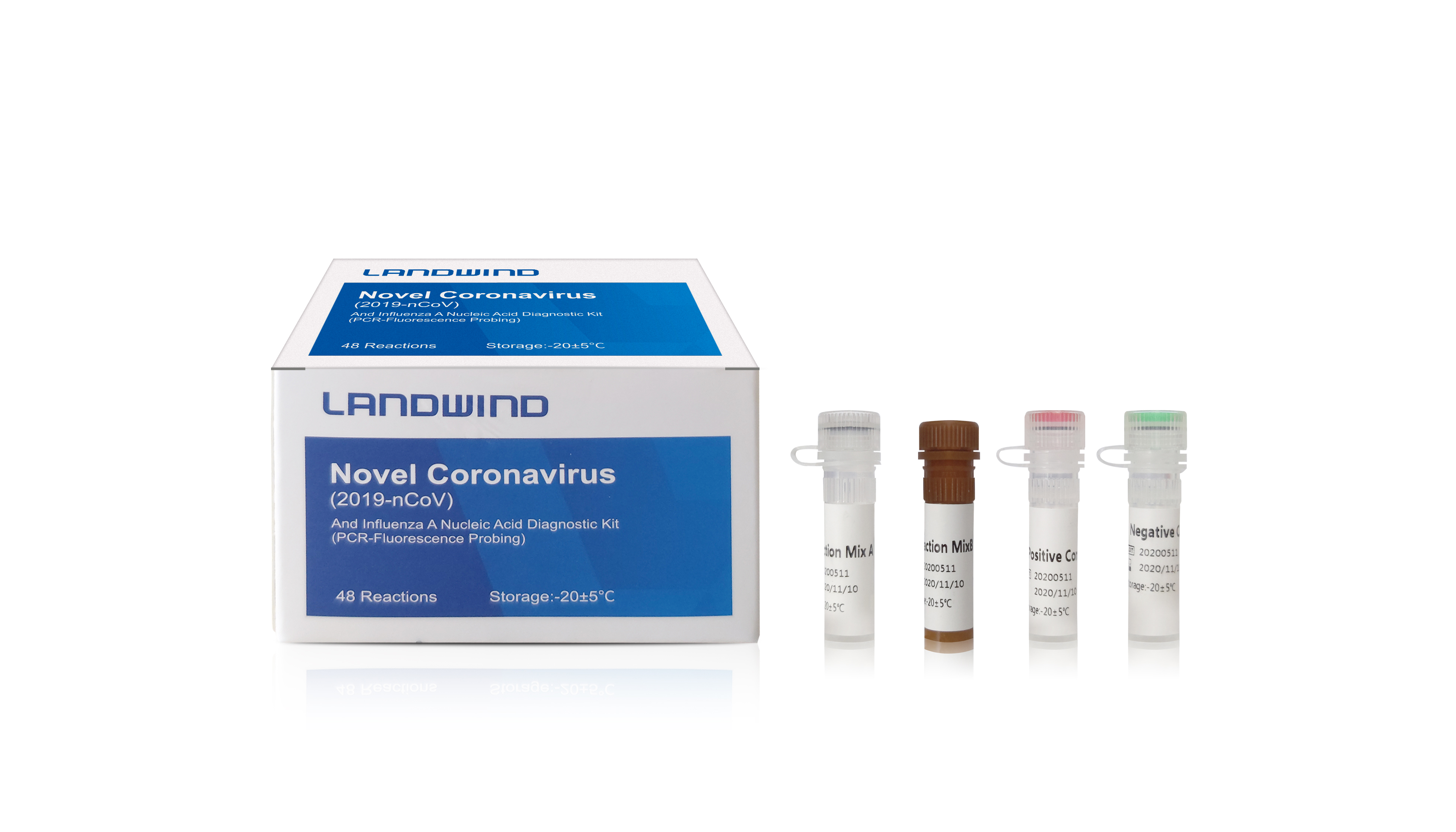
The genetic material of COVID-19 is RNA. The principle of the RT-PCR method is the specific match between the primers and probes and the specific region of COVID-19 RNA. Once the RNA of the virus is detected, the result will be "positive". In terms of Methodology, the nucleic acid detection technology is the most direct and the most intrinsical pathogenicity evidence. It is better than prognosis basing on experience, such as clinical manifestation, clinical characteristics and CT scanning.